Running a gel, thus perfect time to blog. Perhaps if I write stuff in the lab, it may actually turn out to be marginally intellectual.
Hopefully my gel won't turn out to be something like this again:

I hate genotyping - the work itself requires the intelligence of a brain-dead foetal monkey, yet manages to find a stunning variety of ways to fail. And when it does work, it usually shows everything was wildtype anyway. Or maybe your mutant-specific primers failed. You should probably remember to run a positive control next time. If you have one, that is. And when it does work, you can brag about find a homozygous line amongs the seeds you ordered from the stock centre. Nature-worthy achievements right there.
Actually, most lab work is about as mundane and intellectually uninvolving. The planning and literature reading, data analysis and troubleshooting(!) can be quite exciting and tend to involve plenty of thinking, but you only do that in-between the endless repetitive pipetting and mixing chemicals and following protocols. I think research probably involves one of the biggest intellectual (and emotional, heh) rollercoasters of all professions, which is why only the very insane can handle it.
[did I run the DNA off the gel yet... no, phew.]
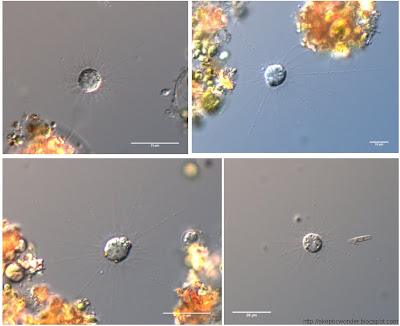
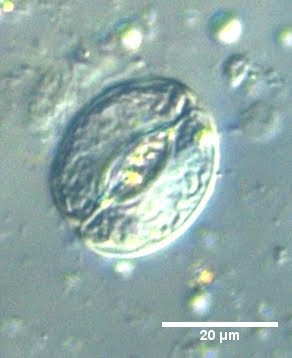
I do enjoy microscopy, however, which is considered by some to be repetitive and mindless as well. I cannot disagree more. It's an artistic break from the gels and protocols - you have a lot of factors to play around with to obtain the best image you can. Microscopy is also very dangerous, a wonderful way to obtain false positives! It's extremely easy to tweak the image to see what isn't there, even unintentionally. A substantial component of the art is to obtain sexy images without sacrificing the scientific validity thereof.
As children, many of us have dreamt of fantasy alien worlds, to be much disappointed upon finding out we would most likely never encounter any; not within our lifetimes anyway. However, it is wonderful to know that alien worlds are not confined to what lies beyond the Earth's atmosphere! (or present time...) They are all around us, ready to provide enchantment and fantasy to anyone who invests enough to explore.
To a mathematician, beauty may lie in quantifying forms or dynamic phenomena, or working with abstract worlds far beyond the imaginative capacity of the human mind. To a fiction writer, fantasy worlds thrive in his mind not much different from the form taken by reality itself. To an astronomer, the alien worlds are almost within reach - just barely visible, yet not ours to manipulate. For me, foreign worlds exist under the lens of a microscope, where if you magnify anything far enough, you can spend a lifetime wandering amidst the surreal landscapes unseen by naked eye. The life of or within a cell is fascinating enough conceptually, but that mysterious aspect of it being beyond our 'scale' makes it not much different from a childhood fairytale or a sci-fi novel - except that it's real!
On a more abstract level, science itself is a foreign world for exploration (along with math, the arts, etc.). Upon liberation from what Carl Sagan calls "the anaesthetic of familiarity", the mundane everyday world around us becomes full of tantilising mystery. Science is a way of unraveling some of this mystery (I'll argue,
the optimal way of dealing with natural mystery), an ardurous journey through the unknown worlds. It's rather fractal-like in nature - the more you explore, the more questions arise; the deeper something is examined, the more detail beckons attention.
I think a good analogy for the scientific journey could be hiking or climbing - the act of walking along a trail is quite repetitive: all you do is place one foot before the other. After some time the scenery begins to repeat itself, and one wonders how anyone could enjoy outdoor activities at all. But many do, and probably few ever notice the monotony of walking, or climbing, or paddling a kayak... for there's always something fresh to look at, something wonderful to appreciate and enjoy. It's fairly easy to knock off the 'anaesthetic of familiarity' for an outdoor enthusiast - the escape from the chaos of the human 'world' is sufficient to make the journey worthy of the physical investments. The threat of an occasional failure, the pain of stepping on a too-unstable rock... is usually not enough to deter one from actually enjoying the exploration itself.
Science is quite similar - there is the monotony and rigorous exactness required by the scientific method, the endless protocols, the multitudes of failed and inconclusive experiments. There is the threat of losing one's job upon failure of publication - the world of 'publish or perish'. It seems one must be insane to voluntarily engage in such activity, much less enjoy it. But just like the monotony of walking is instantly forgotten upon the sight of a rare bird or an overpowering landscape, the monotony of science is instantly lifted upon the sight of a contradicted null hypothesis, or the sight of a rare species, or the ability to actually see a cellular process in action in real time... (perhaps for a chemist, the synthesis of a brand new chemical never before produced on earth could induce a similar rush or euphoria?)
But just as one does not go on a hike expecting to see a rare bird (lest the foiled expectations lead to a wrecked mood), one cannot go into science expecting to make great discoveries. The very process itself must be tolerable, and hopefully captivating enough, to keep going. Sometimes you get excited about the potential outcomes of an experiment, only to discover your results are utterly inconclusive and the story is far more complex than previously imagined. I think what makes science quite suitable for an insane type of mind is the ability to actually not mind such disappointments, and savour them as a testament to nature's superiority and infinite complexity.
After all, we may rest assured that we will never run out of work to do, just as an outdoorsman would never run out of places to explore. Now isn't that a comforting form of eternity?
[genotype results: all wildtype. Time for more PCR!]